- Have any questions?
- 91-22-23726950
- 91-22-23774610
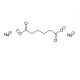
Sodium Adipade
May 10, 2019
Sodium Chloride
May 10, 2019Sodium Bromide
Muby Chemicals established in the year 1976, is pioneer in Manufacturing Chemicals for Oil and Gas Exploration, Hydraulic Fracturing (Fracking) and coiled tube Chemicals.Our advanced chemistry leading to an innovative and high-performance product range is coupled with effective on and off site management services.
We are manufacturer of Specialty chemicals, Pharmaceutical Excipients, Fragrance & Flavorchemicals in India, which are of IP, BP, USP, Ph. Eur., FCC or Food Grade, ACS, AR or Analytical Reagent Grade, LR or Laboratory Reagent Grade, Pure and Technical Grades of various chemicals.
| Sodium Bromide Pure | Our Guaranteed Specs. |
| Sodium Bromide % by wt. of Dried Material | 99% Sodium bromide |
| Chlorides % by Wt. max. | 00.3% |
| Bromates % by Wt. max. | Passes |
| Heavy Metals Wt. max. | Passes |
| Iron % by Wt. max. | Passes |
| Sulphate | Passes |
| Alkali % by Wt. max. | Passes |
| Arsenic | 10 ppm max |
| Barium | Passes the test |
| Moisture % by Wt. max | As agreed |
Sodium Bromide General & Uses
Sodium bromide is a salt with the formula NaBr, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries. Its action is due to the bromide ion (potassium bromide is equally effective). It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion.
Principal chemical reactions
NaBr is widely used in organic synthesis as a nucleophile to convert organochlorine compounds to organobromine derivatives, which are more usefully (selectively) reactive.
NaBr + RCl → RBr + NaCl
Sodium bromide can be used as a source of the chemical element bromine. This can be accomplished by bubbling chlorine gas through an aqueous solution of NaBr.
As a source of HBr, NaBr is treated with a strong, non-volatile acid:
NaBr + H3PO4 → HBr + NaH2PO4
HBr can also be oxidized to Br2 using MnO2 or concentrated H2SO4.
Other Applications
Used as a hypnotic, anticonvulsant, and sedative in medicine. As a source of the bromide ion, which is pharmacologically active, it is equivalent to potassium bromide (see this article for more complete discussion of this topic).
In photography
Used to establish a bromide ion reserve in a bromine spa (hot tub) antimicrobial treatment regimen.




