- Have any questions?
- 91-22-23726950
- 91-22-23774610
Lactobionic Acid
May 7, 2019
Lithium Citrate
May 7, 2019Lactic Acid
Muby Chemicals established in the year 1976, is pioneer in Manufacturing Chemicals for Oil and Gas Exploration, Hydraulic Fracturing (Fracking) and coiled tube Chemicals.Our advanced chemistry leading to an innovative and high-performance product range is coupled with effective on and off site management services.
We are manufacturer of Specialty chemicals, Pharmaceutical Excipients, Fragrance & Flavorchemicals in India, which are of IP, BP, USP, Ph. Eur., FCC or Food Grade, ACS, AR or Analytical Reagent Grade, LR or Laboratory Reagent Grade, Pure and Technical Grades of various chemicals.
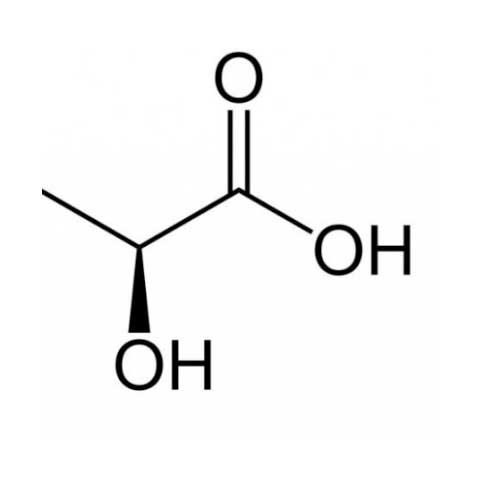
Lactic acid CH3CH(OH)CO2H is a white, water-soluble solid or clear liquid that is produced both naturally and synthetically. Lactic acid is choral, consisting of two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. A mixture of the two in equal amounts is called DL-lactic acid.
Lactic Acid USP Grade
Propanoic acid, 2-hydroxy-. Lactic acid [50-21-5].
Lactic Acid is a mixture of lactic acid (C3H6O3) and lactic acid lactate (C6H10O5) equivalent to a total of not less than 88.0 percent and not more than 92.0 percent, by weight, of C3H6O3. It is obtained by the lactic fermentation of sugars or is prepared synthetically. Lactic Acid obtained by fermentation of sugars is levorotatory, while that prepared synthetically is racemic. [NOTE—Lactic Acid prepared by fermentation becomes dextrorotatory on dilution, which hydrolyzes L-( )-lactic acid lactate to L-(+)-lactic acid.]
Labeling: Label it to indicate whether it is levorotatory or racemic.
Identification: It meets the requirements of the test for Lactate.
Specific rotation: between 0.05 and +0.05 , for racemic Lactic Acid.
Readily carbonizable substances: Rinse a test tube with sulfuric acid, and allow to drain for 10 minutes. Add 5 mL of sulfuric acid to the test tube, carefully overlay it with 5 mL of Lactic Acid, and maintain the tube at a temperature of 15 : no dark color develops at the interface of the two acids within 15 minutes.
Residue on ignition: not more than 3 mg, from a 5-mL portion (0.05%).
Sugars: To 10 mL of hot alkaline cupric tartrate add 5 drops of Lactic Acid: no red precipitate is formed.
Chloride: To 10 mL of a solution (1 in 100) acidified with nitric acid add a few drops of silver nitrate: no opalescence is produced immediately.
Sulfate: To 10 mL of a solution (1 in 100) add 2 drops of hydrochloric acid and 1 mL of barium chloride: no turbidity is produced.
Heavy metals: 0.001%.
Limit of citric, oxalic, phosphoric, or tartaric acid: To 10 mL of a solution (1 in 10) add 40 mL of calcium hydroxide, and boil for 2 minutes: no turbidity is produced.
Assay: To about 2.5 mL of Lactic Acid, accurately weighed in a tared 250-mL flask, add 50.0 mL of 1 N sodium hydroxide, and boil the mixture for 20 minutes. Add phenolphthalein, and titrate the excess alkali in the hot solution with 1 N sulfuric acid. Perform a blank determination.
Each mL of 1 N sodium hydroxide is equivalent to 90.08 mg of C3H6O3.
Lactic Acid FCC Food Grade
α-Hydroxypropionic Acid; 2-Hydroxypropionic Acid
C3H6O3 — Formula wt 90.08
INS: 270 CAS: L(+)-Lactic Acid [79-33-4]
CAS: DL-Lactic Acid [598-82-3]
DESCRIPTION
Lactic Acid occurs as a colorless or yellow, syrupy liquid consisting of a mixture of lactic acid (C3H6O3) and lactic acid lactate (C6H10O5). It is obtained by the lactic fermentation of
sugars or is prepared synthetically. It is usually available in solutions containing the equivalent of from 50% to 90% lactic acid. It is hygroscopic, and when concentrated by boiling, the acid condenses to form lactic acid lactate, 2-(lactoyloxy)propanoic acid, that on dilution and heating, hydrolyzes to Lactic Acid. It is miscible with water and with alcohol.
Function: Acidifier.
REQUIREMENTS
Identification: A sample gives positive tests for Lactate.
Assay: Not less than 95.0% and not more than 105.0% of the labeled concentration of C3H6O3.
Chloride: Not more than 0.1%.
Citric, Oxalic, Phosphoric, or Tartaric Acid: Passes test.
Cyanide: Not more than 5 mg/kg.
Iron: Not more than 10 mg/kg.
Lead: Not more than 0.5 mg/kg.
Residue on Ignition: Not more than 0.1%.
Sugars: Passes test.
Sulfate: Not more than 0.25%.
For Original Monographs of IP Indian Pharmacopoeia BP British Pharmacopoeia USP US Pharmacopoeia FCC Food Grade product, please check with the respective web-pages or books.